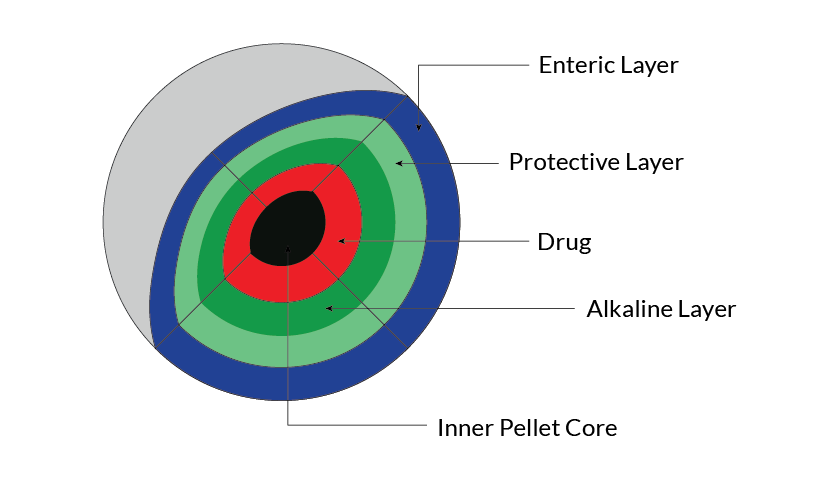
Pharmaceutical Pellets are small, free-flowing, spherical particulates manufactured by the agglomeration of a fine powder, granules of drug substances and excipients using appropriate processing equipment. Pelletization and Granulation are often used interchangeably. However, pelletization technique is considered superior to most techniques that fall under a similar category.
Pellets have undoubtedly become a very important type of dosage form in pharmaceuticals over at least the past two decades or more. Although it was primarily the PPI Inhibitors launched in pellets; other therapeutic categories too quickly found pellet-based drug formulations a place in their dosage form regimen. Thus, Pelletization technology has gained an irreplaceable and permanent place in the NSOSD area of pharmaceutical manufacturing, especially in the last couple of decades.
A host of pelletization equipment and technology is now available routinely for the manufacturing pharmacists to employ for pharmaceutical pellets production. Yet, the area of problem-solving / troubleshooting has been more often than not, an exercise of "shooting in the dark" and with the added "blindfolded" to boot!!
With other dosage forms like Tablets, it is far easier to evaluate tablet coating texture, uniformity, and the effect of the applied coating on the tablet surface and the core matrix itself. However, a similar outcome impossible while considering an evaluation of pellets. The reasons are obvious as listed here.
- Every individual coated unit's size is much smaller than a small-sized tablet and impossible to be assessed by a naked eye examination.
- The number of individual coated units is significantly larger the number of tablets for a given size of a single batch in weight.
Although the size poses a weighty challenge in pellet coating, the sheer number of units that are being coated also pose additional difficulties in assessing the applied coating. For that matter, the success of the whole operation, perse!
This indicates that the evaluation techniques used to assess/predict pellet performance may need to be vastly different from those used for another dosage form like Tablets / Capsules / Powders. The purpose here is to highlight this basic feature and examine the various evaluation methods available for pellets.
Evaluation Methods:
Microscopic examination of the pellets:
Microscopic examination of the pellets is critical to understanding the dosage form performance's process effects for pronounced reasons.
- Core Pellet surface appearance (smooth or rough edges seen)
- Coated pellets surface appearance (absence or presence of abnormalities)
- Cross-section of the pellets (both core and coated pellets)
Core pellet surface appearance needs to be smooth as opposed to having a rough appearance. This will ensure proper application of coating material that will not be prone to cracks or lack in continuity which will go a long way in controlled release characteristics.
SEM of a coated pellet cross-section shows the drug core (crystalline centre) and the translucent polymer coating. Magnification: 250,000
Drug Powder characteristics like surface area, particle size distribution and the effect of the drug layering process on these parameters and the adhesion nature of the drug powder to the NPS seed surface are also key to how the drug release is affected. These parameters need careful monitoring to understand the relationship between them and the drug release in the final dosage form. Further, the initial NPS seed material's quality and size spread is also a very important feature to consider. The trick is to choose the closest size range of the NPS and hence predictable surface area calculations leading to the calculation of the exact polymer requirement since it is a function of the available surface area. Similarly, using coating polymer, solvent, and the amount of polymer and the other excipients added therein also greatly influences the drug release.
The remarkable relationship between the equipment used, batch size, and the stress applied on the pellets and NPS during the coating process be it drug layering or controlled released coatings is noteworthy and missing it would be an understatement. To illustrate, let us suppose that the drug is crystalline. Consequently, it may be possible that some drug particles may shred from the drug layered pellets during subsequent coating and deposit on the coating itself, thus marring the drug release profile!! (However, this may be considered a scale-up problem as one may not experience it during smaller sized trials).
Interestingly, for example, in powder layering using Roto Insert Equipment, there have been instances where, as the process is scaled up, the stress on the drug particle dusted is created due to the higher rotating bed weight. This results in a reduction of the particle size and elimination of sharp edges causing a further reduction in polymer coating applied to match the drug profile of the earlier smaller trial lots where such stress factors are absent!
The key to having control over the pelletization process is having evaluation methods which are ·
- Use a higher sampling method to evaluate pellet/drug particle size nature – The use of Laser techniques for particle size evaluation is a great assistance.
- Scanning electron microscopy (SEM) of the coated surface and a cross-section to show the core and the coating would be of immense help in understanding what is happening in the process
- Applying the above techniques to all stages of the process from starting materials through drug layering and subsequent coatings is often the only way to understand whatever is happening clearly and the resultant drug release seen.
- Other evaluation methods too that need to be followed are several notable amongst which are
- Uniformity of drug content and assay
- Weight gain Reconciliation
- Moisture content
- Pellet size variation (closer range always preferred)
- Bulk density and other physicals (more to strengthen the belief that process is reproducing itself)
- Multiple disso tests from different samples of the same batch is very essential
- A Logbook of observations made during developmental lots and scaled up lots are sacrosanct too
We have discussed only a few techniques here. The article emphasizes the importance of magnification and intense sampling which is the key, in comparison with the usual evaluation methods generally employed. The author believes that some of these may even be employed as routine in-process QC tests for commercial lots to 'ensure reproducibility of results' and hassle-free pellet processing.
RAM GULWADY
Chief Technology Advisor
FABL PROCESS TECHNOLOGIES